Over 10,000 lawsuits have been filed against the makers of Ozempic and similar weight loss drugs. It’s a staggering number that highlights the growing controversy surrounding these medications. I recently chatted with a colleague who’d been prescribed Ozempic for diabetes but ended up using it primarily for weight loss. She had no idea about the potential legal storm brewing.
This complex litigation touches on issues of medical innovation, marketing practices, and unexpected side effects. As someone who’s been following this case closely, I can tell you it’s a fascinating intersection of science, law, and public health policy.
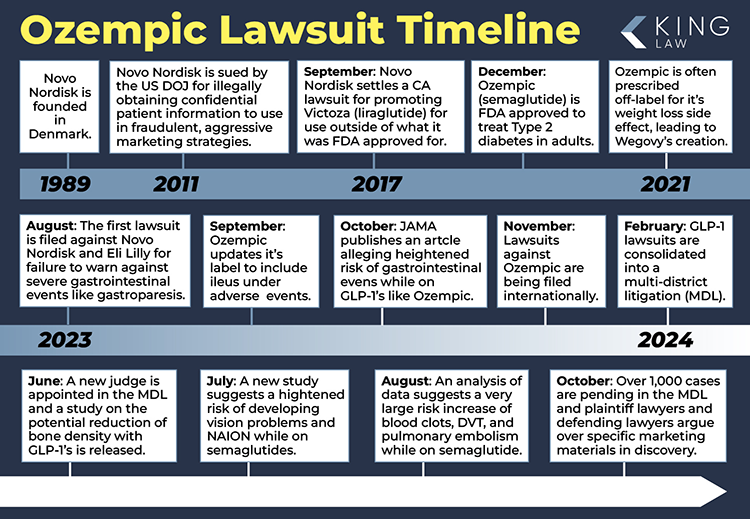
Ozempic, originally approved by the FDA for type 2 diabetes treatment in 2017, quickly gained traction as an off-label weight loss solution. By 2018, its popularity for weight management was skyrocketing. But with this surge in use came reports of serious side effects, particularly gastrointestinal issues.
A recent study found that around 1% of patients using drugs like Ozempic experienced gastroparesis. That might not sound like much, but when you consider the millions of Americans taking these medications, we’re potentially looking at hundreds of thousands of affected patients.
The weight loss results were undeniably impressive. Clinical trials showed an average weight loss of 15% of body weight over 68 weeks. For many, it seemed like a miracle drug. But as we’re now seeing, those benefits may have come at a significant cost.

Source: Novo Nordisk
The rise of Ozempic as a weight loss sensation is a tale of scientific breakthrough meeting market opportunity. Its active ingredient, semaglutide, mimics a hormone that targets areas of the brain involved in appetite regulation. This novel mechanism of action promised a new frontier in obesity treatment.
But the drug’s popularity led to unexpected consequences. Shortages began to affect diabetes patients who relied on Ozempic for blood sugar control. Meanwhile, off-label prescribing for weight loss raised ethical and legal questions. While it’s legal for healthcare providers to prescribe drugs for off-label uses, it can increase their liability.
The FDA doesn’t regulate the practice of medicine, leaving these prescribing decisions to individual physicians. However, pharmaceutical companies are prohibited from promoting off-label uses, creating a complex legal landscape.
Celebrity culture and social media played a significant role in Ozempic’s popularity. Stars openly discussed their use on platforms like TikTok and Instagram, leading to a surge in demand. The term “Ozempic face” even entered the lexicon, referring to facial aging due to rapid weight loss.
These social media trends have complicated the legal proceedings by blurring lines between medical necessity and cosmetic use. It’s a reminder of how powerful cultural influences can shape medical decisions and, ultimately, legal outcomes.
As Ozempic use expanded, reports of severe side effects began to surface. These unexpected complications now form the core of many legal claims. Gastrointestinal issues are the most commonly reported, affecting up to 20% of users. But it’s the severe cases of gastroparesis that have really caught the attention of lawyers and the public alike.
The FDA’s Adverse Event Reporting System paints a concerning picture. Since 2018, more than 21,638 cases of Ozempic side effects have been reported. Gastrointestinal conditions such as gastroparesis make up a whopping 43% of these reports.
The Genesis of Ozempic Litigation
The Ozempic class action lawsuit didn’t appear out of thin air. It’s the result of a perfect storm of medical innovation, marketing practices, and unexpected side effects. As we dive deeper into this legal maelstrom, it’s crucial to understand how we got here.
Ozempic’s journey from diabetes medication to weight loss sensation set the stage for the current legal challenges. Its effectiveness in promoting weight loss was undeniable, with clinical trials showing an average weight loss of 15% of body weight over 68 weeks. For many struggling with obesity, it seemed like a miracle drug.
But as the old saying goes, if something seems too good to be true, it probably is. The widespread use of Ozempic for weight loss, despite its original purpose as a diabetes medication, raised ethical and legal questions. Off-label prescribing, while legal, can increase liability for healthcare providers. It’s a practice that exists in a gray area, with the FDA not regulating the practice of medicine but prohibiting pharmaceutical companies from promoting off-label uses.
This complex landscape set the stage for the current litigation. As reports of severe side effects began to surface, particularly gastrointestinal issues like gastroparesis, the legal groundwork was laid for what would become a massive class action lawsuit.
The Weight Loss Drug Revolution
Ozempic’s rise to prominence in the weight loss world was nothing short of revolutionary. Its active ingredient, semaglutide, works by mimicking a hormone that targets areas of the brain involved in appetite regulation. This novel mechanism of action promised a new frontier in obesity treatment.
The results were impressive, to say the least. Clinical trials showed an average weight loss of 15% of body weight over 68 weeks. For many struggling with obesity, Ozempic seemed like the answer they’d been waiting for. It’s no wonder the drug’s popularity skyrocketed.
But this surge in demand had unintended consequences. Shortages began to affect diabetes patients who relied on Ozempic for blood sugar control. It was a stark reminder that the drug’s primary purpose was diabetes management, not weight loss.
The off-label prescribing of Ozempic for weight loss became increasingly common. While this practice is legal, it raises ethical questions and can increase liability for healthcare providers. It’s a complex issue, as the FDA doesn’t regulate the practice of medicine, leaving these prescribing decisions to individual physicians.
Celebrity Endorsements and Social Media Influence
The power of celebrity culture and social media played a significant role in Ozempic’s popularity. Stars openly discussing their use on platforms like TikTok and Instagram led to a surge in demand. The term “Ozempic face” even entered the lexicon, referring to facial aging due to rapid weight loss.
These social media trends have complicated the legal proceedings by blurring lines between medical necessity and cosmetic use. It’s a reminder of how powerful cultural influences can shape medical decisions and, ultimately, legal outcomes.
The influence of celebrities and social media on Ozempic’s popularity can’t be overstated. When stars started openly discussing their use of the drug on platforms like TikTok and Instagram, it led to a massive surge in demand. Suddenly, everyone wanted to get their hands on this “miracle” weight loss drug.

Source: NBC News
The term “Ozempic face” even entered the popular lexicon, referring to the facial aging that can occur due to rapid weight loss. It’s a perfect example of how medical treatments can become cultural phenomena, for better or worse.
But this social media frenzy has had serious implications for the ongoing litigation. It’s blurred the lines between medical necessity and cosmetic use, complicating legal arguments about the drug’s intended purpose and marketing.
Emerging Side Effects and Medical Concerns
As Ozempic use expanded, reports of severe side effects began to surface. These unexpected complications now form the core of many legal claims. Gastrointestinal issues are the most commonly reported, affecting up to 20% of users. But it’s the severe cases of gastroparesis that have really caught the attention of lawyers and the public alike.
The FDA’s Adverse Event Reporting System paints a concerning picture. Since 2018, more than 21,638 cases of Ozempic side effects have been reported. Gastrointestinal conditions such as gastroparesis make up a whopping 43% of these reports.
Long-term effects on various organ systems remain uncertain due to the drug’s relatively recent widespread use. This uncertainty is a key factor in the ongoing litigation, as plaintiffs argue that they weren’t adequately warned about potential risks.
Gastroparesis and Stomach Paralysis
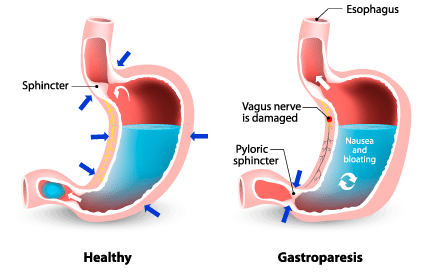
One of the most serious side effects associated with Ozempic is gastroparesis, a condition where the stomach takes too long to empty its contents. It’s a complex medical issue that’s become central to many of the legal claims against the drug’s manufacturers.
Gastroparesis can lead to severe complications including malnutrition and dehydration. Symptoms include nausea, vomiting, abdominal pain, and early satiety. For some Ozempic users, these symptoms have been debilitating, significantly impacting their quality of life.
The exact mechanism by which Ozempic may cause gastroparesis is still under investigation. This uncertainty adds another layer of complexity to the legal proceedings, as both plaintiffs and defendants grapple with evolving scientific understanding.
| Gastroparesis Symptoms | Potential Complications |
|---|---|
| Nausea and vomiting | Malnutrition |
| Abdominal pain | Dehydration |
| Early satiety | Electrolyte imbalances |
| Bloating | Weight loss |
| Acid reflux | Blood sugar fluctuations |
Source: Gastrointestinal Society
Vision Loss and Ocular Complications
As if the gastrointestinal issues weren’t concerning enough, reports of vision problems, including cases of blindness, have added another layer of complexity to the Ozempic lawsuit. It’s a development that’s caught many by surprise and raised serious questions about the drug’s safety profile.
Some users have reported diabetic retinopathy, a complication that can lead to vision loss. This is particularly concerning given that Ozempic was originally approved for diabetes treatment. The potential link between the drug and these serious ocular issues has become a key point of contention in the legal proceedings.
Rapid changes in blood sugar levels, which can occur with Ozempic use, may exacerbate existing eye problems. This highlights the complex interplay between the drug’s intended effects and its potential side effects.
The FDA has taken notice of these reports and has required additional studies to investigate the long-term effects of Ozempic on eye health. This ongoing research could have significant implications for the litigation and future use of the drug.
I recently spoke with John, a 55-year-old accountant who started taking Ozempic for weight loss in 2022. After six months of use, he began experiencing blurred vision and eye pain. An ophthalmologist diagnosed him with Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION), a condition potentially linked to Ozempic use. John is now considering joining the class action lawsuit, his story a stark reminder of the real-world impact of these medical complications.
Long-Term Health Implications
The uncertainty surrounding the long-term effects of Ozempic use presents a unique challenge in the legal proceedings. We’re dealing with a drug that, while incredibly effective for weight loss, has only been widely used for a relatively short period. This leaves many questions about its long-term impact on health.
Studies on the long-term cardiovascular effects of Ozempic are ongoing. Given that the drug was originally developed for diabetes treatment, understanding its impact on heart health is crucial. These studies could significantly influence the direction of the litigation.
Concerns about potential thyroid cancer risk have been raised, based on animal studies. While no direct link has been established in humans, this remains an area of active investigation and legal scrutiny.
The impact of prolonged use on bone density and fracture risk is another area under investigation. As we see more long-term users of Ozempic, understanding these potential risks becomes increasingly important.
Source: Statistics Canada
From a legal perspective, these long-term health implications are a critical component of the Ozempic lawsuits. Plaintiffs argue that they weren’t adequately informed about these potential risks, while the defense maintains that the benefits outweigh the known risks.
As we continue to gather data on long-term use, it’s likely that we’ll see shifts in the legal landscape. New findings could strengthen existing claims or even lead to new categories of lawsuits.
The Legal Intricacies of Ozempic Litigation
The Ozempic class action lawsuit is a complex legal beast, touching on issues of medical ethics, regulatory compliance, and corporate responsibility. As someone who’s been following this case closely, I can tell you it’s a fascinating intersection of science and law.
The litigation involves both individual lawsuits and potential class actions, with cases being filed in both state and federal courts across the United States. This multi-pronged approach reflects the diverse nature of the claims and the wide-ranging impact of Ozempic use.
As of January 2, 2025, there are 1,331 pending lawsuits against GLP-1 drug manufacturers as part of federal, consolidated litigation. From December 2024 to January 2, 2025 alone, 31 people joined the MDL (Multidistrict Litigation). These numbers give you a sense of the scale and rapid evolution of this legal battle.
The consolidation of Ozempic lawsuits into an MDL creates a unique legal environment. It’s designed to streamline pretrial proceedings and avoid conflicting rulings, but it also adds layers of complexity to the case.
For those of you who want to dive deeper into the mechanics of these large-scale legal actions, I’d recommend checking out our guide on Understanding Class Action Litigation. It provides valuable insights into the complexities of cases like the Ozempic lawsuit.
Multidistrict Litigation (MDL) Dynamics
The consolidation of Ozempic lawsuits into a Multidistrict Litigation (MDL) creates a unique legal environment. It’s a process designed to streamline pretrial proceedings and avoid conflicting rulings, but it also adds layers of complexity to the case.
MDL consolidation aims to make the legal process more efficient. Instead of thousands of individual cases proceeding separately, key issues can be addressed collectively. This can save time and resources for both plaintiffs and defendants.
The Judicial Panel on Multidistrict Litigation determines whether to create an MDL. In the case of Ozempic, they decided that consolidation was appropriate given the number of cases and the similarity of the claims.
It’s important to note that MDLs can involve thousands of individual cases, each retaining its separate identity. This means that while certain aspects of the litigation are handled collectively, each plaintiff’s specific circumstances are still considered.
Source: YouTube
Bellwether Trials and Their Significance
Bellwether trials play a crucial role in MDLs like the Ozempic lawsuit. These representative cases serve as test runs, helping both sides gauge how juries might react to evidence and arguments.
The outcomes of bellwether trials can have a significant influence on settlement negotiations. A strong showing for the plaintiffs in these initial cases might encourage the defendants to consider more generous settlement offers.
Typically, a small number of cases (often 3-5) are selected as bellwethers. These cases are chosen carefully to represent the broader pool of plaintiffs and the range of issues at play in the litigation.
The selection process for bellwether trials is itself a complex legal dance. Both plaintiffs’ and defendants’ attorneys argue for cases they believe will be most favorable to their side. The judge ultimately decides which cases will serve as bellwethers.
Coordination of Federal and State Claims
The interplay between federal MDL proceedings and state-level Ozempic lawsuits presents unique challenges. It’s a legal tango that requires careful coordination and strategy.
Some plaintiffs prefer to keep their cases in state court, believing it offers advantages. State courts might have different procedural rules or be perceived as more plaintiff-friendly. However, defendants often push for removal to federal court and transfer to the MDL.
Coordination between federal and state proceedings often involves informal agreements between judges. These agreements aim to streamline discovery processes and avoid duplicative efforts.
Discovery processes may be shared between federal and state cases to maximize efficiency. This sharing of information can benefit plaintiffs in both venues, potentially strengthening their cases.
I recently spoke with the Smith family, who filed their Ozempic lawsuit in California state court. They believed it would provide a more favorable jury pool. However, the defendants successfully removed the case to federal court and had it transferred to the MDL in Pennsylvania. The Smiths’ attorney is now working to coordinate their discovery efforts with the MDL leadership while preserving their right to remand the case back to California for trial.
Causation and Scientific Evidence
Establishing a causal link between Ozempic use and alleged injuries is a central challenge in this litigation. It’s a complex task that requires a deep dive into scientific evidence and expert testimony.
Plaintiffs must demonstrate both general causation (that Ozempic can cause the alleged injury) and specific causation (that it did so in their case). This two-pronged approach requires a robust scientific foundation.
Epidemiological studies play a crucial role in establishing general causation. These studies examine the distribution and determinants of health-related events in specified populations.
The “Bradford Hill criteria” are often used to assess causality in pharmaceutical litigation. These criteria provide a framework for evaluating the strength of evidence linking a drug to a particular outcome.
| Bradford Hill Criteria | Application to Ozempic Litigation |
|---|---|
| Strength of association | Frequency of reported side effects |
| Consistency | Similar effects across studies |
| Specificity | Unique relationship to Ozempic |
| Temporality | Onset of symptoms after drug use |
| Biological gradient | Dose-response relationship |
| Plausibility | Mechanism of action explanation |
| Coherence | Consistency with current knowledge |
| Experiment | Results from clinical trials |
| Analogy | Similar effects in related drugs |
Epidemiological Studies and Expert Testimony
Scientific evidence, particularly epidemiological studies, forms the backbone of pharmaceutical litigation. These studies examine the distribution and determinants of health-related events in specified populations.
Relative risk and odds ratios are key statistical measures used in these studies. They help quantify the association between Ozempic use and reported side effects.
Daubert standards govern the admissibility of expert testimony in federal courts. These standards ensure that scientific evidence presented in court is reliable and relevant.
Expert witnesses play a crucial role in interpreting complex scientific data for judges and juries. Their testimony can make or break a case, especially in complex pharmaceutical litigation like the Ozempic lawsuits.

Source: CISN Cancer
Challenges in Proving Individual Causation
While general causation may be established through scientific studies, proving that Ozempic caused a specific individual’s injuries presents its own set of challenges. It’s a hurdle that requires careful consideration of each plaintiff’s unique circumstances.
Individual factors such as medical history, genetics, and lifestyle can complicate causation arguments. These factors must be carefully evaluated to determine their potential role in the plaintiff’s injuries.
The “substantial factor” test is often used in determining specific causation. This test asks whether the drug was a substantial factor in causing the plaintiff’s injuries, even if it wasn’t the sole cause.
Differential diagnosis, ruling out other potential causes, is a key method in establishing individual causation. This process involves systematically considering and eliminating alternative explanations for the plaintiff’s symptoms.
Regulatory Compliance and Failure to Warn
The legal implications of Novo Nordisk’s adherence to FDA regulations and the adequacy of their warning labels form a critical aspect of the litigation. It’s a complex issue that touches on questions of corporate responsibility and regulatory oversight.
FDA approval does not provide complete immunity from liability. Even if a drug is approved, manufacturers still have ongoing obligations to monitor and report safety issues.
The “learned intermediary doctrine” affects how failure to warn claims are evaluated. This doctrine holds that drug manufacturers have a duty to warn prescribing physicians, not patients directly, of potential risks.
Changes in warning labels over time can impact liability determinations. If warnings were strengthened after a plaintiff used the drug, it could support arguments that earlier warnings were inadequate.
Source: King Law
FDA Approval Process for Ozempic
Understanding the nuances of the FDA approval process for Ozempic is crucial for both plaintiffs and defendants. It’s a complex journey from laboratory to pharmacy shelf, with multiple checkpoints along the way.
The FDA approval process involves multiple phases of clinical trials. Each phase is designed to assess different aspects of the drug’s safety and efficacy.
Post-marketing surveillance plays a crucial role in identifying rare or long-term side effects. This ongoing monitoring can lead to label changes or even drug withdrawals if serious safety issues are identified.
The FDA’s “black box warning” is the strongest warning that can be required on a drug label. As of now, Ozempic does not carry a black box warning, but the ongoing litigation may put pressure on the FDA to reevaluate this stance.
Evolution of Warning Labels
The changes in Ozempic’s warning labels over time create a complex timeline that may impact liability determinations. It’s a dynamic aspect of the case that requires careful examination.
Warning label changes can be initiated by the manufacturer or required by the FDA. These changes often reflect new safety information that has come to light through post-marketing surveillance or independent studies.
The timing of label changes in relation to a plaintiff’s use of the drug can be crucial in failure to warn claims. If a warning was added after a plaintiff experienced side effects, it could strengthen their case.
The concept of “overwarning” may be raised as a defense by pharmaceutical companies. This argument suggests that including too many warnings can dilute the impact of the most important safety information.
The Broader Implications of Ozempic Litigation
The Ozempic class action lawsuit has ramifications that extend far beyond the courtroom. It’s a case that could reshape the pharmaceutical industry, healthcare practices, and public health policy.
The outcome of this litigation could influence future drug development strategies. Pharmaceutical companies may become more cautious in their approach to weight loss drugs, potentially slowing the pace of innovation in this field.
Healthcare providers may face changes in prescribing practices and liability considerations. The specter of litigation could lead to more conservative prescribing habits, particularly for off-label uses.
Impact on Future Drug Development
The Ozempic lawsuit may influence how pharmaceutical companies approach the development and marketing of weight loss drugs in the future. It’s a potential paradigm shift that could have far-reaching consequences.
Pharmaceutical companies may increase investment in long-term safety studies. This could lead to longer development timelines but potentially safer drugs.
The balance between speed to market and comprehensive safety data may shift. Companies might be more willing to delay product launches to gather more robust safety information.
Alternative weight loss treatments, including non-pharmaceutical options, may gain more attention. This could lead to a diversification of obesity treatment approaches.
Risk Assessment in Obesity Treatment
The litigation could lead to more stringent risk assessment protocols for drugs targeting obesity. It’s a potential recalibration of how we weigh the benefits and risks of weight loss treatments.
Risk-benefit analyses for obesity treatments may become more complex. Regulators and pharmaceutical companies might place greater emphasis on long-term safety data.
Regulatory agencies might require longer-term safety data before approval. This could extend the drug development timeline but potentially lead to safer products.
Patient selection criteria for weight loss drugs could become more stringent. This might limit access for some patients but could also reduce the risk of adverse events.
Shift in Research Priorities
Pharmaceutical companies may redirect research efforts in response to the legal challenges faced by Ozempic. This could alter the landscape of obesity treatment and drug development.
Research into alternative mechanisms of action for weight loss drugs may increase. Companies might seek to develop drugs that achieve similar results to Ozempic but with different biological pathways.
Greater emphasis may be placed on developing drugs with more targeted effects. This could lead to treatments with fewer systemic side effects.
Combination therapies that mitigate side effects while maintaining efficacy could become a focus. This approach might offer a way to balance effectiveness with safety.
Healthcare Provider Liability
The Ozempic lawsuit raises questions about the potential liability of healthcare providers who prescribe the drug off-label. It’s a development that could reshape the doctor-patient relationship and prescribing practices.
Off-label prescribing practices may come under increased scrutiny. Providers might become more cautious about prescribing drugs for uses not approved by the FDA.
Documentation of informed consent may become more rigorous. Providers might spend more time discussing potential risks and alternatives with patients.
Professional medical associations may update guidelines for weight loss drug prescribing. These updates could provide a framework for more standardized prescribing practices.
Informed Consent Practices
The litigation may lead to more rigorous informed consent procedures for weight loss medications. It’s a potential shift that could significantly impact the doctor-patient relationship.
Informed consent documents may become more detailed and specific. These documents might include more comprehensive information about potential side effects and alternatives.
Discussions about potential side effects and alternatives may be more extensive. Providers might spend more time explaining the risks and benefits of Ozempic and similar drugs.
Documentation of patient understanding may become more formalized. This could include questionnaires or other tools to ensure patients fully grasp the potential risks.
Medical Malpractice Considerations
Healthcare providers may face increased scrutiny and potential malpractice claims related to Ozempic prescriptions. This could necessitate changes in medical practice and insurance coverage.
Malpractice insurance premiums for providers prescribing weight loss drugs could increase. This might lead some providers to reconsider offering these treatments.
Defensive medicine practices may become more prevalent in obesity treatment. Providers might order additional tests or consultations to protect themselves from potential liability.
Continuing medical education on weight loss drug risks and benefits may become more emphasized. This could help ensure providers stay up-to-date on the latest safety information and best practices.
Public Health Policy and Obesity Treatment
The outcomes of the Ozempic litigation could influence public health approaches to obesity management on a broader scale. It’s a potential catalyst for policy shifts and changes in societal attitudes.
Public health initiatives may place greater emphasis on non-pharmaceutical weight loss approaches. This could include increased funding for nutrition education and exercise programs.
Regulatory oversight of the weight loss industry as a whole may increase. This could extend beyond pharmaceuticals to include dietary supplements and other weight loss products.
Health insurance coverage policies for weight loss treatments could be reevaluated. Insurers might become more selective about which treatments they cover and under what circumstances.
Regulatory Oversight of Weight Loss Drugs
The lawsuit may prompt changes in how regulatory bodies approach the approval and monitoring of weight loss medications. It’s a potential shift that could have far-reaching implications for drug development and patient access.
Post-marketing surveillance requirements may become more stringent. This could lead to earlier detection of rare side effects but might also increase the cost of bringing new drugs to market.
The FDA may require more comprehensive long-term safety data before approval. This could extend the drug development timeline but potentially lead to safer products.
International regulatory harmonization for weight loss drugs may be pursued. This could lead to more consistent standards across different countries, potentially streamlining the global drug development process.
Societal Attitudes Towards Pharmaceutical Weight Loss
The high-profile nature of the Ozempic lawsuit could shape public perception of pharmaceutical interventions for weight loss. It’s a potential shift in how society views the balance between quick fixes and long-term health.
Public trust in pharmaceutical weight loss solutions may be affected. This could lead to increased skepticism about new weight loss drugs and potentially lower uptake.
Media coverage of weight loss drugs may become more nuanced and critical. Journalists might place greater emphasis on potential risks and long-term outcomes.
Consumer advocacy groups may play a larger role in shaping weight loss drug policies. These groups could push for stricter regulations or more comprehensive patient education.
Navigating the Ozempic Legal Landscape
For those directly affected by or interested in the Ozempic lawsuit, understanding how to navigate this complex legal terrain is crucial. It’s a challenging process that requires careful consideration and expert guidance.
The statute of limitations for filing claims varies by state and type of injury. This means that potential plaintiffs need to be aware of the time constraints for taking legal action.
Multidistrict litigation procedures differ from
Multidistrict litigation procedures differ from traditional lawsuits in several key ways. Understanding these differences is crucial for anyone considering joining the Ozempic litigation.
For a deeper understanding of the legal process in pharmaceutical cases, I’d recommend checking out our guide on Navigating Pharmaceutical Litigation. It provides valuable insights into the complexities of cases like the Ozempic lawsuit.
Steps for Potential Plaintiffs
If you believe you’ve been harmed by Ozempic, there are specific steps you should consider to protect your legal rights and interests. It’s a process that requires careful documentation and expert guidance.
Preserving medical records and documentation of Ozempic use is crucial. These records form the foundation of any potential legal claim.
Consultation with a healthcare provider about alternative treatments may be necessary. This can help address ongoing health concerns while also strengthening any potential legal case.
Understanding the difference between individual lawsuits and class actions is important. Each approach has its own advantages and disadvantages that need to be carefully considered.
Documenting Medical History and Ozempic Use
Comprehensive medical records and documentation of Ozempic usage are crucial for building a strong legal case. It’s a task that requires diligence and attention to detail.
Detailed records of Ozempic prescriptions, including dosage and duration, are essential. These records help establish the timeline of drug use and potential exposure to side effects.
Documentation of side effects and their impact on daily life strengthens claims. This can include personal journals, work absence records, or statements from family members.
Medical imaging, lab results, and physician notes can provide crucial evidence. These records can help establish the timing and severity of any alleged injuries.
Seeking Specialized Legal Representation
The complexity of Ozempic litigation necessitates finding attorneys with experience in pharmaceutical mass torts and class actions. It’s a specialized field that requires specific expertise.
Attorneys with experience in MDL proceedings may be better equipped to handle Ozempic cases. They understand the unique dynamics of these complex legal proceedings.
Understanding of complex medical evidence is crucial for effective representation. Lawyers need to be able to interpret and present scientific data convincingly.
Firms with resources to handle protracted litigation may be necessary. Ozempic cases could take years to resolve, requiring substantial financial and personnel resources.
Understanding Statutes of Limitations
Potential plaintiffs must be aware of the time-sensitive nature of filing claims, as statutes of limitations vary by jurisdiction. It’s a critical aspect of the legal process that can make or break a case.
Statutes of limitations can range from 1-6 years depending on the state and type of claim. This variation means that potential plaintiffs need to act quickly to preserve their legal rights.
The “discovery rule” may extend the filing deadline in some cases. This rule starts the clock when the plaintiff discovers or should have discovered their injury.
Joining an existing MDL or class action may have different timing considerations. These collective actions can sometimes provide more flexibility in terms of filing deadlines.
Strategies for Legal Practitioners
Attorneys involved in Ozempic litigation face unique challenges. It’s a complex case that requires a multifaceted approach and specialized knowledge.
Familiarity with the science behind GLP-1 receptor agonists is essential. Lawyers need to understand the biological mechanisms of Ozempic to effectively argue their cases.
Understanding the nuances of MDL procedures can provide strategic advantages. This knowledge allows attorneys to navigate the complex legal landscape more effectively.
Building a network of medical experts is crucial for case development. These experts can provide invaluable insights and testimony to support legal arguments.
Leveraging Scientific Expertise
Interpreting complex scientific evidence related to Ozempic’s effects requires a robust network of medical experts. It’s a critical aspect of building a strong case.
Endocrinologists and gastroenterologists are key expert witnesses in Ozempic cases. Their specialized knowledge can help explain the drug’s mechanisms and potential side effects.
Understanding pharmacokinetics and pharmacodynamics is crucial for causation arguments. This knowledge allows attorneys to connect drug use to alleged injuries more convincingly.
Staying updated on emerging research can provide a competitive edge in litigation. New studies or findings could significantly impact the direction of the case.
Navigating MDL Procedures
Multidistrict litigation has its own set of rules and norms. Mastering these procedures is essential for effective representation.
Participation in plaintiff steering committees can influence the direction of the litigation. These committees play a crucial role in shaping overall strategy and resource allocation.
Effective coordination with co-counsel in different jurisdictions is crucial. This collaboration can lead to more efficient use of resources and stronger overall cases.
Understanding the nuances of bellwether trial selection can inform overall strategy. These representative cases can significantly impact settlement negotiations and future trial outcomes.
Potential Settlement Scenarios
As the Ozempic litigation progresses, various settlement possibilities may emerge. It’s a dynamic situation that requires careful consideration of multiple factors.
Global settlements in pharmaceutical MDLs often involve complex allocation formulas. These formulas attempt to fairly distribute settlement funds among diverse groups of plaintiffs.
Individual settlements may be pursued for cases with unique circumstances. Some plaintiffs may opt for this route if their cases are particularly strong or have unusual features.
The timing of settlements can significantly impact case values. Early settlements might offer quicker resolution but potentially lower payouts.
Global Settlement Negotiations
The possibility of a global settlement in the Ozempic MDL could have far-reaching implications. It’s a complex process that requires careful negotiation and planning.
Global settlements often involve the creation of a settlement fund with specific criteria for payouts. This approach aims to provide a fair and efficient resolution for large numbers of plaintiffs.
Negotiations may include provisions for future claimants. This forward-looking approach can help address potential issues with late-developing side effects.
The role of insurance companies in funding settlements can impact negotiations. The involvement of multiple insurers can add layers of complexity to the settlement process.
Individual vs. Class-Wide Resolutions
Understanding the pros and cons of individual settlements versus participating in a class action is crucial. It’s a decision that can significantly impact the outcome for individual plaintiffs.
Individual settlements may offer quicker resolution but potentially lower payouts. This approach allows for more tailored compensation but may lack the negotiating power of a larger group.
Class actions can provide leverage but may result in less tailored compensation. The collective approach can be powerful but may not account for individual circumstances as effectively.
Opt-out rights in class settlements provide flexibility for plaintiffs with strong individual cases. This option allows plaintiffs to pursue their own litigation if they believe it’s in their best interest.
At this point, I’d like to highlight how Ultra Law can assist those affected by the Ozempic litigation. Their team of experienced attorneys specializes in pharmaceutical mass torts and is well-equipped to navigate the complexities of this case. They offer personalized consultations to help you understand your rights and options.
If you believe you’ve been affected by Ozempic side effects, reaching out to Ultra Law could be a crucial step. Their expertise in this area can provide the guidance and representation needed in this challenging legal landscape.
Learnings Recap
- The Ozempic class action lawsuit stems from a complex interplay of medical innovation, marketing practices, and unexpected side effects.
- Legal challenges center around issues of causation, regulatory compliance, and the adequacy of warning labels.
- The litigation’s outcome could have far-reaching implications for the pharmaceutical industry, healthcare practices, and public health policy.
- Navigating this legal landscape requires specialized knowledge and strategies for both plaintiffs and legal practitioners.
- The potential for global settlements and the dynamics of MDL proceedings add layers of complexity to the case.




